41°C Avian Soluble Collagen
An Expert of Skincare and Repair
Hanson biomedical
AGEs Immunoassay
Autologous Stem Cell

Hanson Pet Family
Visualine II® Dual Occult Blood FaecoCheck Test
FaecoCheck test is able to distinguish the bleeding from Lower GIT or Upper GIT in the routine screening.
Cat. No: VH-010 (100 tests/kit) - Patent Pending
Lateral Flow, Membrane Based Monoclonal Immunoassay for Qualitative Detection of Human Haemoglobin in Faeces
![]() INTENDED USE
INTENDED USE
The Hanson® Faecocheck Test is an easy to use, contains of both immuno-test and chemical test. The monoclonal immunoassay is major for the Lower GIT screening and designed to provide a qualitative analysis of human haemoglobin in faecal samples. The chemical test is major for the Upper GIT screening and designed to provide a qualitative analysis of human heme in faecal samples. The Faecocheck Test combined immunoassay and chemical test in the same device as an all-in-one faecal occult blood screening test. The test is intended for use by medical laboratories and physicians. The assay should not be attempted without proper supervision and is not intended for over the counter sale to laypersons.
The Hanson® Faecocheck Test is a screening test providing a preliminary analytical test result only. It should be used in conjunction with other tests to properly exclude the presence of gastrointestinal tract (GIT) pathologens. Any positive results should be promptly followed up using more definitive tests such as colonoscopy or barium enema X-ray.
![]() PRINCIPLE OF TEST
PRINCIPLE OF TEST
The Hanson® Faecocheck Test is an affordable, qualitative screening test used to identify individuals, asymptomatic or otherwise, for further investigative testing. The detection of faecal occult blood who will need a indicates GIT bleeding. Although some daily blood loss to the GIT is normal (1), excessive bleeding is an indicator of a range of pathological conditions from insignificant to potentially fatal. It is through the early detection of faecal occult blood that the effects and progress of serious pathologies such as polyps, diverticulae, ulcers and colorectal cancer may be reduced or removed completely.
The Hanson® Faecocheck Test combined immunoassay and chemical test in the same device. The immunological aspect of the Hanson® Faecocheck Test uses a membrane bound monoclonal anti-human haemoglobin antibody to specifically bind human haemoglobin in faecal samples. The specificity of the antibody for human haemoglobin eliminates the possibility of false positive results stemming from heme(hematin), dietary sources of haemoglobin and dietary peroxidases.
Once bound to the monoclonal antibody the hemoglobinРђЎs pseudoperoxidase activity is utilized to produce a blue coloured end product. The reaction involves the oxidation of alpha guaiaconic acid by the catalytic action of haemoglobin in the presence of peroxide. The product of the reaction appears as a localized blue line on the test strip. The appearance of such product constitutes a positive reaction. If no haemoglobin is present in the sample or the amount present is less than the minimum detection limit of the test, no coloured end product will form and the result is deemed negative. The immunoassay is major for the Lower GIT screening although some of haemoglobin will through stomach into intestinal tract (2).
The chemical aspect of the Hanson® Faecocheck Test is designed to detect human heme that digests from upper GIT bleeding. It also uses the pseudoperoxidase activity of heme groups to produce a blue coloured end product. However, without the ability to selectively bind human haemoglobin, all haemoglobins (human or animal), partially denatured haemoglobins, and individual heme molecules will produce this coloured end product. This lack of specificity, which is so often the cause of false positives particularly when one uses the chemical testing method alone, is used by the Hanson® Faecocheck Test to indicate bleeding originating from the upper GIT. That is, by being able to detect the presence of heme groups and other products of human haemoglobin that have been partially digested by the stomach and the test can compare the bleeding originated from the Upper GIT with whole haemoglobin associated with lower GIT bleeds.
![]() REAGENTS/MATERIALS PROVEDED
REAGENTS/MATERIALS PROVEDED
Each Hanson® Faecocheck Test kit contains:
- 100 Hanson® Faecocheck test strips.
- One 6ml dropper bottle of alpha guaiaconic acid reagent in aqueous ethanol.
- One 6ml dropper bottle of Hydrogen peroxide reagent in aqueous ethanol.
- 100 applicators.
- 100 plastic zip-lock bags (Option).
- 100 Droppers.
- Product insert
- Optional: gloves
![]() WARNINGS AND PRECAUTIONS
WARNINGS AND PRECAUTIONS
FOR IN-VITRO DIAGNOSTIC USE ONLY
- Avoid cross contamination of samples by using a new sample applicator for each test.
- Do not use kits beyond their expiry date.
- Faecal samples and all materials coming into contact with them should be handled and disposed of as if infectious.
- alpha guaiaconic acid and hydrogen peroxide are skin and mucosa irritants. Should contact occur wash affected area thoroughly with water.
- The alpha guaiaconic acid and hydrogen peroxide solutions contain ethanol and as such are flammable and subject to evaporation.
- Alpha guaiaconic acid and hydrogen peroxide are both light-sensitive and should not be exposed to light.
![]() STORAGE
STORAGE
Hanson┬« Faecocheck Test kits are best stored at 2-8РёЃ. (Storage at ambient room temperature less than 30РёЃ is also permissible for up to 2 weeks).
![]() SPECIMEN COLLECTION AND HANDLING
SPECIMEN COLLECTION AND HANDLING
If using the Applicator Stick to apply a faecal sample directly to the sample application well, best results are obtained using a faecal collector(container) or bag to collect the stool. Only a small sample of the stool is required for the test but it must be a representative sample. For best results the sample should be obtained from at least two parts of the stool, ideally the inner and outer areas to maximize the likelihood of detecting any blood.
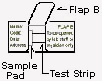
If using the dropper to apply an aqueous sample, simply draw up a dropper full of water (distilled water) into the faecal container and mix well with stool specimen. Open flap A on the Faecocheck test slide, then adds 5 drops of the stool-water suspension solution into the sample application well. Allow time for all solution to be absorbed.
All samples should be applied to the Hanson® Faecocheck strip as soon as possible to avoid degradation of haemoglobin.
As GIT bleeding is often intermittent, three consecutive stool samples should be examined using one Hanson® Faecocheck slide per sample.
![]() ASSAY PROCEDURES
ASSAY PROCEDURES
A) AT THE HOME
PROCEDURE FOR USING THE APPLICATOR STICK:
Patient to complete steps 1 - 7; Physician to complete steps 8 - 12.
- IMPORTANT - Allow the test device to come to room temperature prior to use then tear open the foil sachet and remove the contents.
- Label the Hanson® Faecocheck Test strip appropriately.
- Use the sample applicator to obtain a representative sample of the stool .That is, collect a small amount of feaces from at least two parts of the stool, ideally inner and outer areas.
- Open Flap A on the front of the Faecocheck test slide and apply the faecal sample to the sample application well using the sample applicator stick. Apply the sample such that the sample application well is approximately 80% covered and permeated with faecal matter.
- Add 5 drops of water(distilled water) to the well. Allow time for all water to be absorbed.
- Close flap A and place the test strip in the zip lock plastic bag provided.
- Send the Faecocheck test to your doctor, a medical laboratory or nurseРђЎs station immediately.
- Ensure the patient sample was applied at least 10 minutes before read the Faecocheck slide. (Tests may be used up to 14 days after application of the sample).
- Ensure the Hanson® Faecocheck test strip is correctly labeled and all reagents have reached room temperature before proceeding.
- Open Flap B on the back of the Faecocheck test to expose the development area.
- Add one drop of alpha guaiaconic acid solution followed by one drop of hydrogen peroxide solution to each test strip and sample pad.
- Read results 3 to 5 minutes after application of the hydrogen peroxide solution.
B) IN THE LABS
PROCEDURE FOR USING THE DROPPER:
Patient to complete steps 1 ; Physician to complete steps 2 - 9.
- Collected the stool (0.125 g or more) by the container and return to the Lab by the patients.
- Allow the test device to come to room temperature prior to use then tear open the foil sachet and remove the contents (IMPORTANT).
- Label each of Hanson® Faecocheck Test strip appropriately for each of sample collectors.
- Draw up a dropper full of distilled water (approx. 1ml) into the faecesРђЎ container and mix well with stool specimens.
- Open flap A on the Faecocheck test slide and uses same dropper to add 5 - 6 drops of the stool-water suspension solution into the sample application well. Allow time for all solution to be absorbed.
- Close flap A and allow the test to stand for 10 minutes (tests may be used up to 14 days after application of the sample).
- Open Flap B on the back of the Faecocheck test to expose the
development area.
Add one drop of alpha guaiaconic acid solution followed by one drop of hydrogen peroxide solution to each test strip and sample pad. - Read results 3 to 5 minutes after application of the hydrogen peroxide solution.
![]() INTERPRETATION OF RESULTS
INTERPRETATION OF RESULTS
Results are to be read 3 to 5 minutes after the addition of hydrogen peroxide solution to the test strip. Any significance colour development occurring after 5 minutes is irrelevant and has no influence on the test result interpretation.
Immunological method (for Lower GIT Screening)
A positive result indicates the presence of human haemoglobin in the faecal sample and presents as the formation of a blue line in the test strip area.
A negative result is obtained when there is no haemoglobin in the sample or the amount present is below the lower detection limit of the test. No blue coloured end product forms in the test strip area when a result is negative.
Chemical method (for Upper GIT Screening)
A positive result indicates the presence of whole haemoglobin, components of haemoglobin an/or haem groups all of which may be of human or animal/dietary origin. A positive result is represented by a generalized distribution of blue coloured end product over the sample pad area as well as on the absorbent membrane in the sample application well.
A negative result is obtained when no haemoglobin, haemoglobin digests or haem groups are present in the sample or they are present at concentrations below the detection limit of the test. No blue coloured end product forms in the sample pad area for the negative sample.
| 1. | Immuno-(Negative) Test Strip has no blue line. Chemical-(Negative) Sample Pad is not forming blue colour. |
| Fig. 1 |
 |
| 2. | Immuno-(Positive) Test Strip has a blue line. Chemical-(Positive) Sample Pad is forming blue colour. |
| Fig. 2 |
 |
| 3. | Immuno-(Negative) Test Strip has no blue line. Chemical-(Positive) Sample Pad is forming blue colour. |
| Fig. 3 |
 |
| 4. | Immuno-(Positive) Test Strip has a blue line. Chemical-(Negative) Sample Pad is not forming blue colour. |
| Fig. 4 |
 |
![]() QUALITY CONTROL
QUALITY CONTROL
Quality control testing should be carried out whenever a batch of patient samples is run. Hanson Hong Biomedical is able to supply the positive and negative controls. Alternatively one can use an in house positive control as follows:
- Draw a small volume of whole normal blood into an EDTA or heparinised tube.
- Mix the blood well then dilute 1:101 (e.g. 10╬╝l + 1ml) with distilled water.
- Open Flap A and apply 4 to 5 drops of dilute blood to the sample application well. Allow all to be absorbed by the well.
- Close the flap and leave to stand for 10 minutes.
- Open Flap B on the back of the test strip and add one (1) drop of alpha guaiaconic acid to each stripРђЎs development disc followed by one (1) drop of hydrogen peroxide solution.
- Read results 3 to 5 minutes after addition of the hydrogen peroxide. A blue line should develop in this time. If no blue product appears all the tests are invalid and must be repeated.
![]() LIMITATIONS
LIMITATIONS
- Not all GIT pathologies cause bleeding and those that do may cause intermittent bleeding. As such the Hanson® Faecocheck Test is not a definitive diagnostic test, rather it is designed to aid diagnosis and should be used in conjunction with other diagnostic procedures to fully discount any GIT disease.
- Certain medications can cause GIT bleeding which may be confused with pathological bleeds. Hence the patientРђЎs history with respect to pharmaceuticals should be known.
- High levels of ascorbic acid may cause false negative results and as such patients taking vitamin C should stop two days prior to doing the test.
- Patients with homozygous haemoglobin S and haemoglobin C are not suitable candidates for the test as the monoclonal antibody used in the test only weakly binds these two forms of haemoglobin.
- As the result of the test is determined by a coloured end changed for result people who are colour-blind or visually impaired should not perform the test.
- People with non-GIT bleeds such as menstrual bleeding, haematuria and hemorrhoids are not suitable candidates for the test. Physicians and laboratory staff with abrasions on their hands must take precautions so as not to contaminate the test strip creating false positive results.
- Blood may not be evenly distributed throughout the stool so it is necessary to obtain the sample for testing from at least two areas of the stool.
- Right procedure step is important for FaecoCheck test. Do not use a commercial collector that contains dilution buffer as an accessory of other test kit to perform FaecoCheck test. Due to different dilution factor, It may occur false negative result.
- To avoid draw mucus from stool suspension because the mucus may impede Sample Pad to absorb the liquid.
- Insure Sample Pad absorbed Guaiaconic acid for the following color development.
![]() PERFORMANCE
PERFORMANCE
The monoclonal antibody used in the Hanson® Faecocheck Test has been tested in-vitro against rabbit, pig, sheep, cow and fish haemoglobin with all producing negative results. Peroxidases derived from vegetables were tested and results were negative. This is due to the high specificity of the antibody for human haemoglobin.
The FaecoCheck test was compared with a commercial available immunoassay (OC-Hemodia, Eiken) for human haemoglobin which has a cut-off value at 0.2 - 0.4 m g/ml. 44 clinical stool samples were evaluated by both systems. Of the 44 samples, 5 were determined positive and 39 were determined negative by the OC-Hemodia method; 6 were determined as positive and 38 were determined as negative by FaecoCheck (immunoassay) method; 34 were determined as positive and 10 were determined as negative by FaecoCheck (chemical assay) method; all of 44 were determined as positive by O-toluidine method. Six positives were proved that is including colorectal cancer 1 caseсђЂmalignant gastrointestinal tumor 1 caseсђЂduodenal ulcer 2 casesсђЂacute gastritis 1 case and acute salmonella infected 1 case. The FaecoCheck test correctly identified 100 % of these positive samples and 100 % of these negative samples. It may be liquid faces' samples to occur 1 false negative from OC-Hemodia. All of 6 positive cases were determined positive by FaecoCheck (chemical assay) method.
![]() REFERENCES
REFERENCES
- Ebaugh FG, Clemens Jr T, Rodnan G, Peterson RE: Quantative measurement of gastrointestinal blood lose. 1. The use of radioactive 51Cr in pateints with gastrointestinal haemorrhage. Am. J. Med. 1958 25, 169-181.
- Nakama H; Kamijo N; Fujimori K; Fattah AS; Zhang B. Diagnostic accuracy of immunochemical faecal occult blood test for gastric cancer. J Med Screen, 3(3):113-4 1996.
- Fath, Jr. R.B., Winawer, S.J. Early Diagnosis of Colorectal Cancer. Ann. Rev. Med. 1983. 34, 501-17.
- Greegor, D.H. Occult Blood Testing for Detection of Asymptomatic Colon Cancer. Cancer. 1971. 28, 131-4.
- Simon, J.B. Occult Blood Screening for Colorectal Carcinoma: A Critical Review. Gastroenterology. 1985. 88, 820-37.
| Cat. No. | Description | Packing |
| VH-010 | FaecoCheck Test | 100 tests/kit |
| VH-010-1 | Reagent 1 & 2 | pair |
| VH-010-2 | Applicator Stick | 100ea/pk |
| VH-010-3 | Dropper | 100ea/pk |
| FC-010 | Positive control (standard Human Hb) | 2 ml/vial |
| FC-011 | Faeces container | 1000ea/pk |